Rate Constant K Difference . the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate. — the rate constant k is independent of the concentration of a, b,. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area.
from www.chegg.com
— the rate constant k is independent of the concentration of a, b,. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. The reaction orders in a rate. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant.
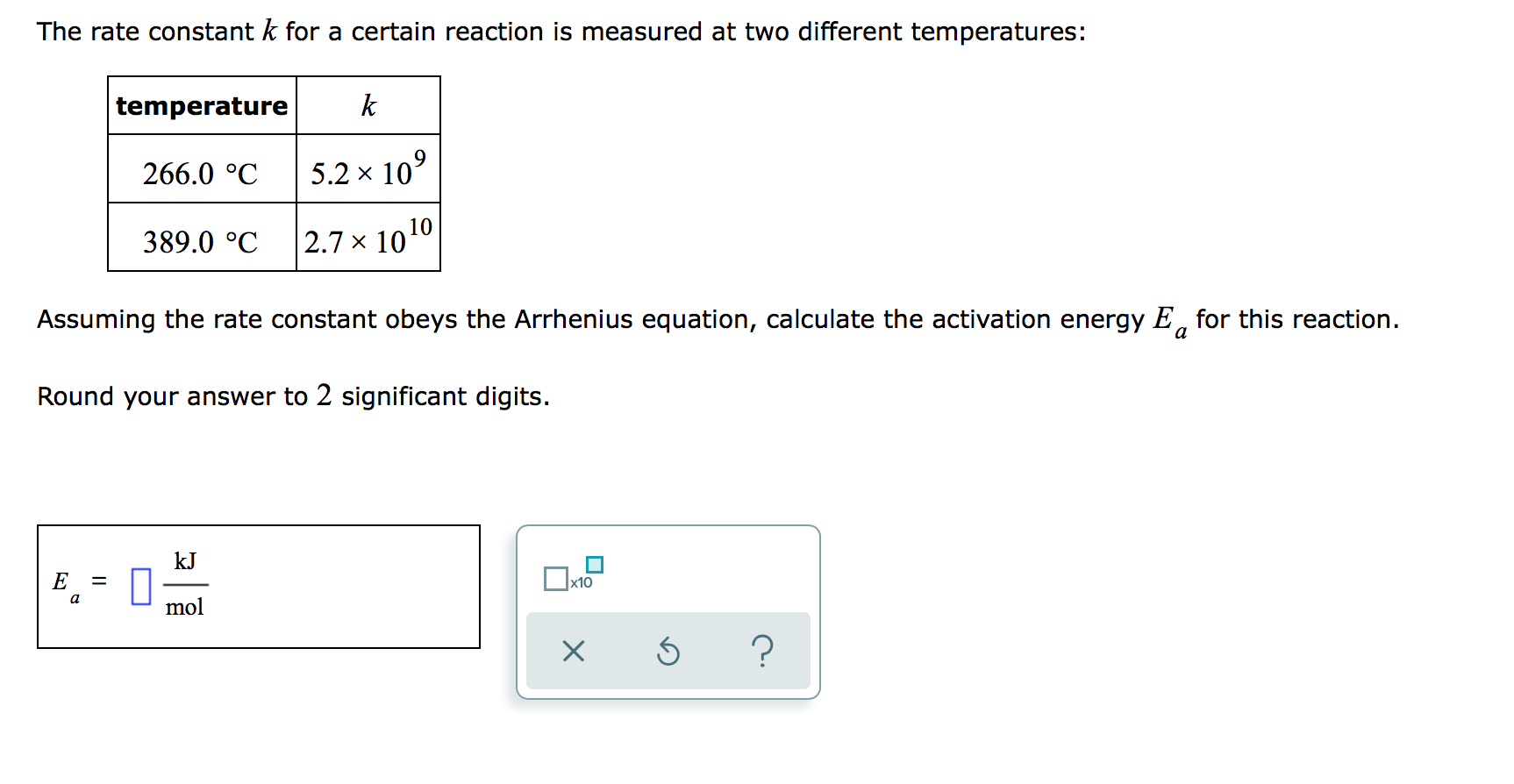
Solved The rate constant k for a certain reaction is
Rate Constant K Difference the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. — the rate constant k is independent of the concentration of a, b,. The reaction orders in a rate. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation ID5609537 Rate Constant K Difference the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant (k) of a rate law is a constant of proportionality between. Rate Constant K Difference.
From www.toppr.com
The rate constants k1 and k2 for two different reactions are 10^16e Rate Constant K Difference the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate. the rate constant (k) of a rate law is a constant of. Rate Constant K Difference.
From www.youtube.com
Relationship Between the Equilibrium Constants YouTube Rate Constant K Difference the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of. Rate Constant K Difference.
From www.researchgate.net
The figures show the relative rate of reaction dependence of the rate Rate Constant K Difference the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant k is independent of the reactant concentrations, but it does vary. Rate Constant K Difference.
From www.researchgate.net
Rate constant k for the reaction CN+NH3 as a function of temperature Rate Constant K Difference the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. — the rate constant k is independent of the concentration of a, b,. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant. Rate Constant K Difference.
From www.slideshare.net
Chapter 15 Lecture Chemical Equilibrium Rate Constant K Difference the rate constant k is independent of the reactant concentrations, but it does vary with temperature. — the rate constant k is independent of the concentration of a, b,. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. The reaction orders in a rate. the. Rate Constant K Difference.
From www.youtube.com
For a reaction, the values of rate constant k at two Rate Constant K Difference the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. — the rate constant k is independent of the concentration of a, b,. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant. Rate Constant K Difference.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate Rate Constant K Difference the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. The reaction orders in a rate. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the rate constant (k) of a rate law is a constant of. Rate Constant K Difference.
From www.researchgate.net
Determination of reaction rate constant (k) for composting at different Rate Constant K Difference The reaction orders in a rate. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k is independent of the concentration of a,. Rate Constant K Difference.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Rate Constant K Difference the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. — the rate constant k is independent of the concentration of a, b,. the rate constant (k). Rate Constant K Difference.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates Rate Constant K Difference the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant k is independent of the reactant concentrations, but it does vary. Rate Constant K Difference.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Rate Constant K Difference the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and. Rate Constant K Difference.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Rate Constant K Difference the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. — the rate constant k is independent of the concentration of a, b,. The. Rate Constant K Difference.
From www.youtube.com
units of the rate constant k derivations YouTube Rate Constant K Difference the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. — the rate constant k is independent of the concentration of a, b,. the rate constant. Rate Constant K Difference.
From educationhealey.z13.web.core.windows.net
How To Calculate Formula Units Chemistry Rate Constant K Difference — the rate constant k is independent of the concentration of a, b,. The reaction orders in a rate. the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of. Rate Constant K Difference.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K Difference the specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations. — the rate constant k is independent of the concentration of a, b,. The reaction orders in a rate. the rate constant k is independent of the concentration of a, b, or c, but it does vary. Rate Constant K Difference.
From www.youtube.com
14.6 Chemical Equilibrium and Rate Constants YouTube Rate Constant K Difference the rate constant k is independent of the concentration of a, b, or c, but it does vary with temperature and surface area. The reaction orders in a rate. — the rate constant k is independent of the concentration of a, b,. the rate constant (k) of a rate law is a constant of proportionality between the. Rate Constant K Difference.
From www.researchgate.net
The secondorder rate constant, k′, as a function of the concentration Rate Constant K Difference — the rate constant k is independent of the concentration of a, b,. the rate constant k is independent of the reactant concentrations, but it does vary with temperature. the rate constant (k) of a rate law is a constant of proportionality between the reaction rate and the reactant. the specific rate constant \(\left( k \right)\). Rate Constant K Difference.